Among the Most Advanced Genetically Engineered Antibody Mouse Models Globally
Among the Most Advanced Genetically Engineered Antibody Mouse Models Globally
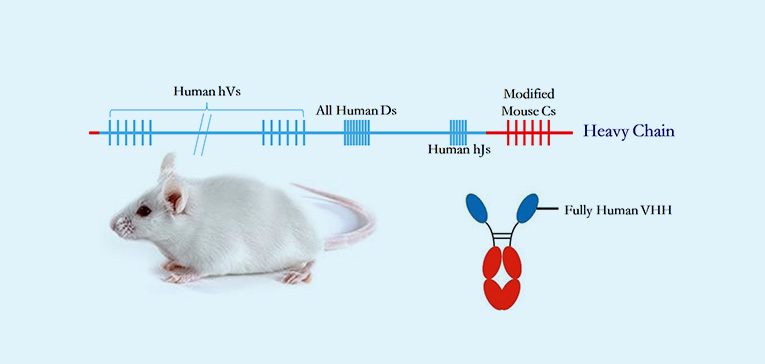
· Three distinct fully human antibody mouse platforms address diverse development needs for monoclonal antibodies (mAbs), bispecifics, and nanobodies, enabling comprehensive support for all fully human antibody-based therapeutics.
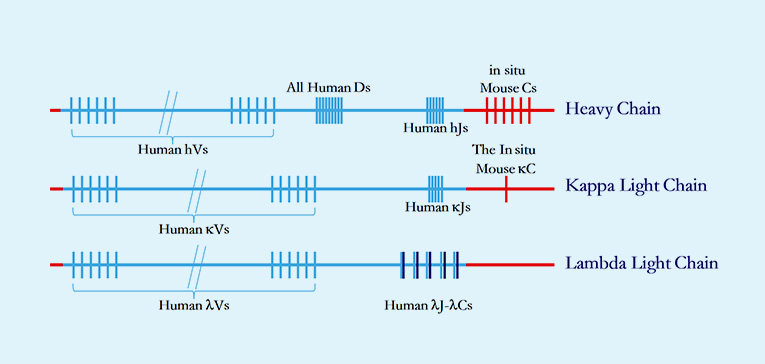
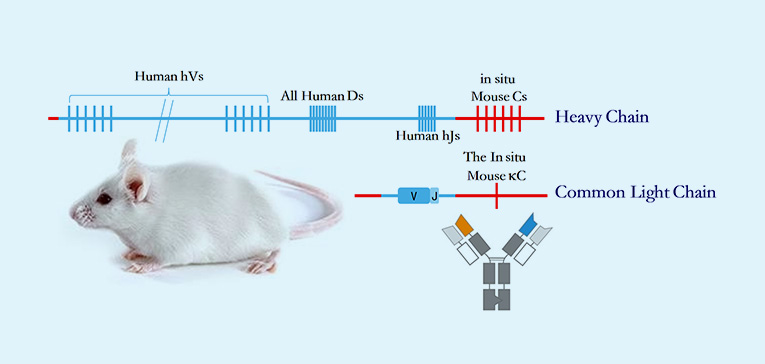
· Unparalleled humanization depth in both heavy- and light-chain variable region gene clusters generates maximal human V(D)J recombination diversity to capture rare epitopes with clinical relevance.
· Site-specific genomic recombination via homologous replacement preserves native murine antibody regulatory elements, ensuring physiological antibody production and GLP-compliant PK profiles.
· Multi-strain compatibility (BALB/c, CD1, SJL) enables immunization optimization across disease models while maintaining wild-type-level antigen-specific responses, validated in oncology, immunology, and CNS programs.