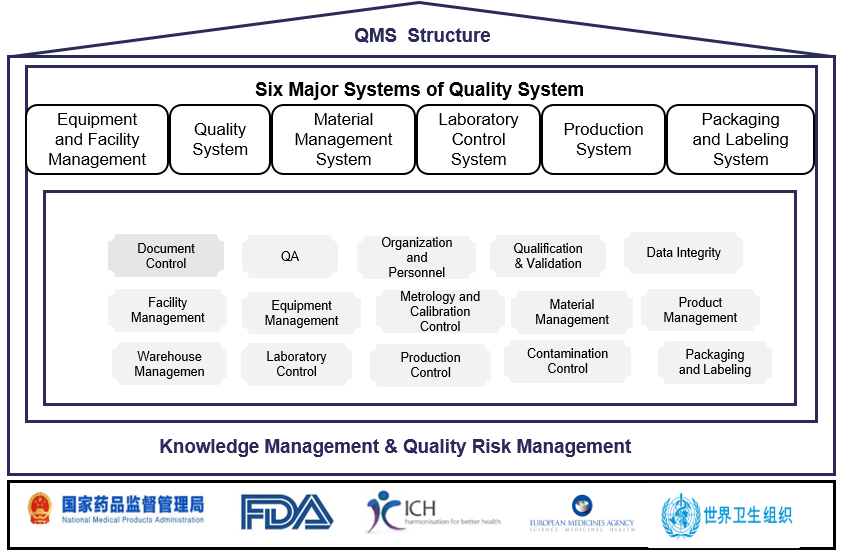
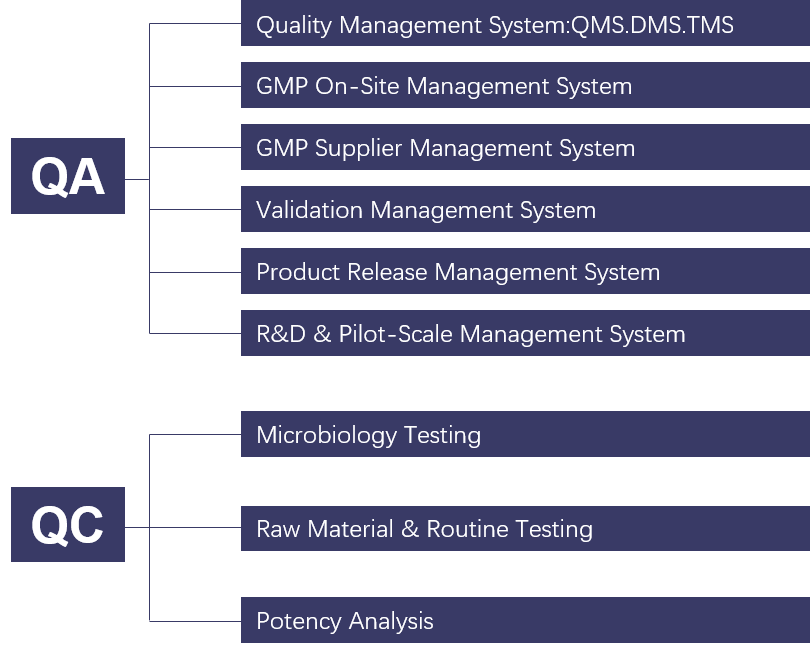
Tarlead Biologics has established a drug production quality management system that meets regulatory requirements such as FDA, EU, NMPA, WHO, etc. The quality system is continuously updated through quality management reviews and continuous improvement procedures. Quality risk management and knowledge management are integrated throughout the entire process of establishing and operating the quality system. Currently, it has successfully supported the application of seven IND projects in China, the United States, and Australia.
The specific quality management system is divided into six major systems, classified as follows: