1、Preparation and evaluation (physicochemical properties, drug release characteristics, blood sample separation, biological distribution, pharmacokinetics characteristics) technology of nano drugs (liposomes, disks, micelles, nanoparticle, nanocrystals, biomimetic nano preparations)
1.1、Nanodrug preparation technology
We have a leading platform for nano drug preparation technology both domestically and internationally, including thin film dispersion, high-pressure homogenization, microfluidics, and microfluidics, to achieve large-scale production of nano drugs from small-scale to pilot scale; Flexible preparation of liposomes, discs, and micelle nanoparticles through phospholipid regulation technology; Construct biomimetic nano formulations using biofilm encapsulation technology and achieve high drug loading and active target molecule modification. The technology platform is used to design and construct various targeted and non targeted liposomes, disks, micelles, nanoparticle, nanocrystals, biomimetic nano preparations, etc. (Figure 1), to meet the packaging needs of small molecule drugs, protein polypeptides and nucleic acid drugs.
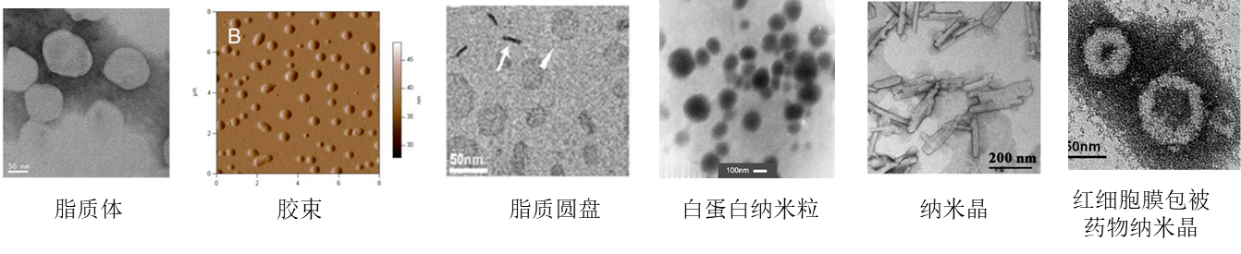
Figure 1. Morphology of nanomedicine
Example: RGD modified red blood cell membrane coated drug nanocrystal delivery system
Chemotherapy drugs were prepared into nanocrystals and incubated with erythrocyte vesicles containing Streptavidin PEG phospholipid material, so that drug nanocrystals were inserted into the erythrocyte membrane of Streptavidin, and then incubated with biotinylation RGD. Through the affinity between biotin and avidin, RGD was modified to the surface of erythrocyte membrane to obtain RGD modified erythrocyte membrane coated drug nanocrystals. The research results show that (Figure 2), the RGD modified erythrocyte membrane coated with drug nanocrystals shows that it has an obvious nuclear membrane structure, and its stability is significantly improved; In vivo pharmacokinetics indicates that biofilm coated drug nanocrystals have the advantage of long circulation, significantly prolonging the drug's circulation time in the body; By using the target molecule modification strategy of Streptomyces avidin, the electrostatic interaction between positively charged RGD peptides and negatively charged red blood cell membranes was shielded, resulting in a good tumor targeting effect; Meanwhile, due to the significant increase in drug loading caused by the use of drug nanocrystals as skeleton materials, significant therapeutic effects were achieved in a single dose.
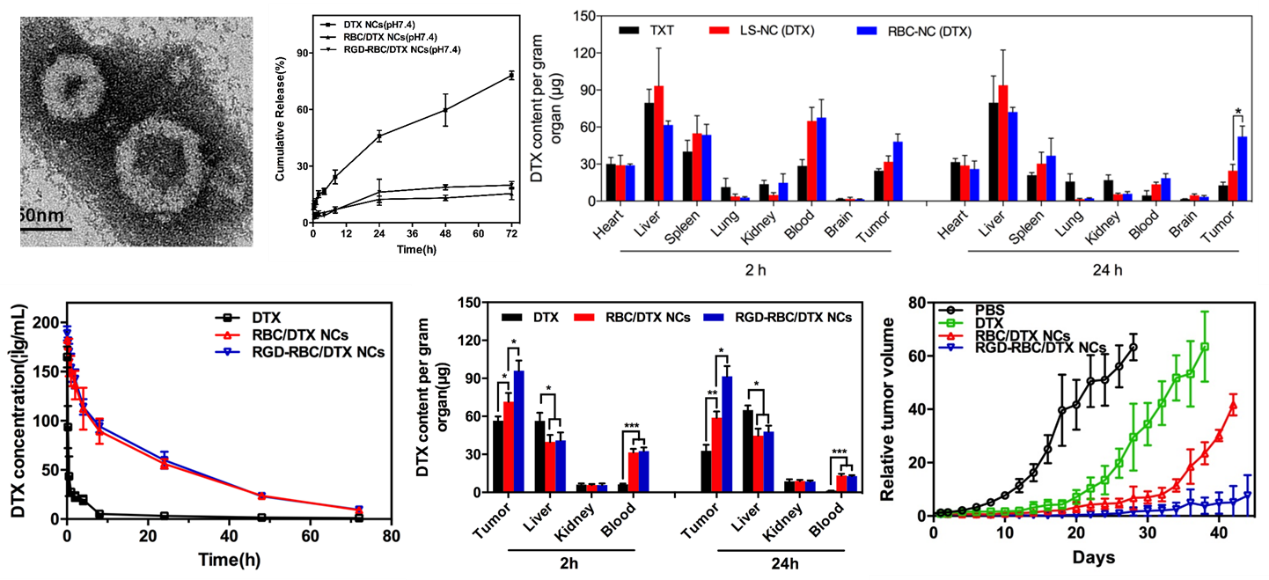
Figure 2. Characterization and efficacy of RGD modified red blood cell membrane coated drug nanocrystals
1.2、Nanodrug evaluation technology
It has a relatively complete technical platform for nano drug evaluation, including: evaluating the particle size, distribution, shape and surface charge of nano drugs through dynamic light scattering, electron microscope, atomic force microscope, etc; Immunogold colloid, Surface plasmon resonance, isothermal titration and nanoflow were used to evaluate the surface modification of nano drugs; Characterization of drug states using X-ray diffraction and DSC; Determination of encapsulation efficiency and drug loading through separation methods such as molecular exclusion chromatography, ultracentrifugation, and ultrafiltration; Using methods such as flow cell and reciprocating dialysis to determine the dissolution and release of nanomedicine, and signing a strategic cooperation agreement with Logan Company to jointly develop quality evaluation technologies such as dissolution and release of high-end drug formulations; Utilizing the independently developed PEG-scFv affinity chromatography method to achieve efficient separation of blood samples for PEG-based nanomedicine; Evaluate the in vivo kinetics and tissue distribution of nanomedicines using methods such as fluorescence in vivo imaging, radioactive tracing, and liquid mass spectrometry; Construct an animal model to evaluate the in vivo efficacy of nanomedicines. We can provide a comprehensive evaluation service for nanomedicine from physical and chemical property identification to in vivo pharmacokinetic and pharmacological evaluation.
Example: PEG-scFv affinity chromatography for efficient blood sample separation of liposomes
This technology utilizes PEG-scFv affinity chromatography (AfC) to efficiently separate blood samples of long-circulating liposomes. Compared with the conventional centrifugation (Centri) method, the AfC method showed a significantly higher liposome recovery rate (10.3 times) (Figure 3).
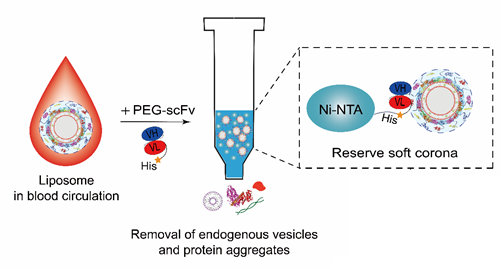
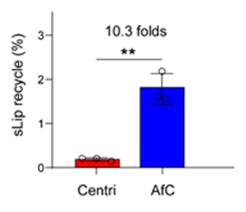
Figure 3. Schematic diagram and recovery rate of blood sample separation process using liposome affinity chromatography
Related patents:
1. Lu Weiyue, Chai Zhilan, Hu Xuefeng, Xie Cao, Hou Huimin, Wang Hao; A preparation method and application of biomembrane encapsulated drug nanocrystals (2017102072756, application date March 31, 2017; PCT/CN2018/081364, application date March 30, 2018; US11260032 B2, authorization date March 1, 2022; 2020-502758, notified by the Japanese Patent Office); Transferred in September 2019, with a transfer amount of 1 million yuan
2. Lu Weiyue, Chai Zhilan, Hu Xuefeng, Wei Xiaoli; A preparation method for actively targeting biofilm nanoparticles (2017101291987, authorized on December 8, 2020); Transferred in September 2019, with a transfer amount of 1 million yuan
3. Lu Weiyue, Hu Xuefeng, Xie Cao; A Novel Phthalic Dihydroxy Polymer Carrier and Its Application in the Construction of Drug Complex Nanodelivery Systems (2017107687598, Authorization Date January 18, 2022)
4. Lu Weiyue, Lu Wuyuan, Chen Xishan, Zhan Changyou, Xie Cao, Yan Zhiqiang; A binding peptide polymer micelle formulation for anticancer use (2015100357530, authorized on June 21, 2019)
5. Lu Weiyue, Li Chong; A drug loaded co delivery lipid nanodelivery system (2009102480426, authorized on August 21, 2013)
6. Zhan Changyou, Zhang Zui, Tang Wenjing, Ying Tianlei, Li Cheng; The Use and Separation Method of PEG-scFv Antibody in the Separation of Free Drugs and PEG Carrier Drugs (2021105154720, Application Date May 12, 2021)
7. Liu Min, Wang Dongli, Song Jie, Wang Jing; A guanidine derivative and its gene delivery system (2020102397783, application date March 30, 2020)
8. Liu Min, Wang Jing, Hu Xuefeng, Wang Dongli, Song Jie, Xie Cao; An imidazole derivative and its gene delivery system (2017108730129, application date September 25, 2017)
9. Lu Weiyue, Wang Songli, Wang Ruifeng, Xie Cao; A Biomimetic Nanodelivery System for Protein Thrombolytic Drugs and Its Application (2019111344848, Application Date November 19, 2019)
10. Ding Jiandong, Chang Guangtao, Lu Weiyue, Li Chong; A polymer and its micelles with pH responsive tumor tissue (2010101320935, application date March 25, 2010)
 Home
>
Business
>
Tayzen
>
Nanodrug Preparation and Evaluation Technology
Home
>
Business
>
Tayzen
>
Nanodrug Preparation and Evaluation Technology











