Evaluation techniques for in vivo processes (protein coronavirus, immunogenicity) of targeted drug delivery systems
1.1. Targeted drug delivery system in vivo protein crown evaluation technology
After entering the body, nano drugs may form protein crowns on their surfaces, change their surface properties and interfere with the recognition of phagocyte, thus changing their behavior in vivo and affecting the efficacy. We can evaluate the composition, formation mechanism, and in vitro and in vivo behavior of targeted drug delivery system protein crowns through methods such as proteomics, cell uptake, pharmacokinetics, tissue distribution, and immune analysis. Furthermore, we have developed a technology for actively targeting protein crowns formed on the surface of nanomedicine by regulating them.
Example: Study on Folate Liposome Protein Crown
Folate liposomes form protein crowns in the blood, and component analysis shows that their main binding protein is natural IgM; The ELISA test showed that natural IgM has strong binding activity with folate, and folate liposomes lose their binding activity to folate receptors after adsorbing protein crowns; In vivo pharmacokinetic and distribution results indicate that folate liposomes are rapidly cleared in the blood, and the clearance rate increases with the increase of folate modification ratio. Folate liposomes are mainly distributed in macrophages in the liver, spleen, and tumors (Figure 8).
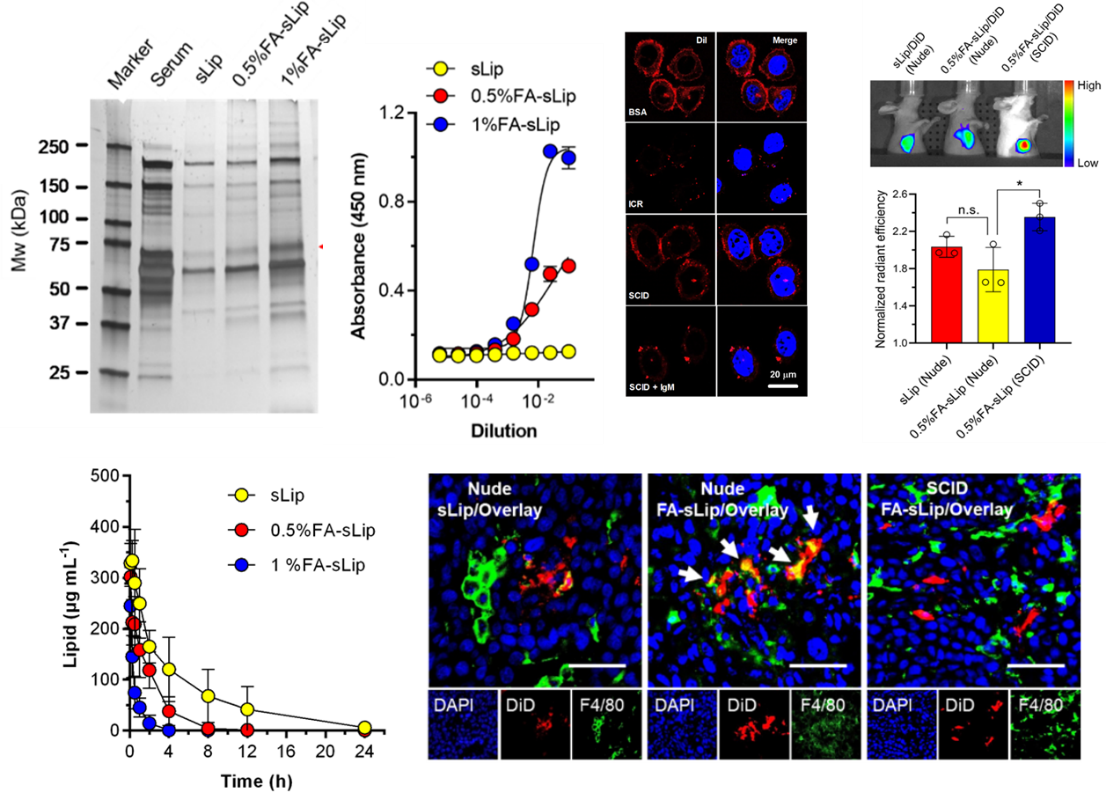
Figure 8. Evaluation of in vitro and in vivo behavior of folate liposome protein crown
Example: A β Modified Liposome Adsorbed Protein Corona Cross BBB Brain Targeting
Will have the function of combining with APOE A β Construction of A by modifying peptide fragments onto liposomes β- After incubating LS with mouse plasma, the main components of its protein crown were analyzed to be APOA4, APOE, and APOA1 from mouse sources; The uptake results of brain capillary endothelial model cells (bEnd. 3) showed that after plasma incubation, A β- The efficiency and fluorescence intensity of LS uptake significantly increased; The pharmacokinetics and distribution results in vivo indicate that A β- The formation of protein crowns in the blood of LS did not accelerate the elimination of liposomes in vivo, and achieved cross BBB brain targeting function (Figure 9).
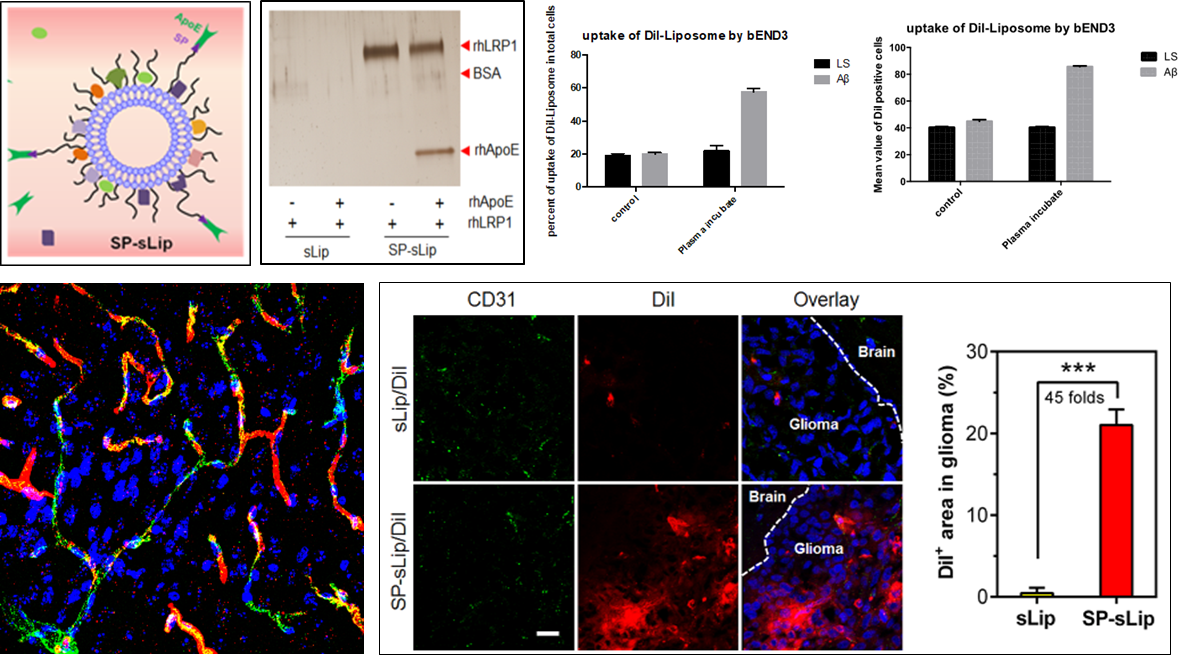
Figure 9. A β- LS adsorption of APOE for brain cross BBB targeting function
Related patents:
Zhan Changyou, Zhang Zui; A type of amyloid protein β Short peptide mediated brain targeted delivery system and its preparation method and use (2018106266524, application date June 14, 2018; PCT/CN2019/087363, application date May 12, 2019)
1.2. Targeted drug delivery system in vivo immunogenicity evaluation technology
We were the first to report the discovery that targeted drug delivery systems can trigger immune responses in the body, leading to a decrease in drug safety and efficacy. In response to this issue, we have developed a method for determining the immunogenicity of targeted drug delivery systems through plasma IgG antibody titer detection, and established an efficient technical platform for evaluating and screening the immunogenicity of targeted drug delivery systems in vivo.
Example: Research on targeted drug delivery systems with no immunogenicity/low immunogenicity
In the study of targeted delivery of low-toxicity molecular targeted drug MTI-31 using c (RGDyK) modified liposomes (c (RGDyK) - liposomes), it was found that c (RGDyK) - liposomes have immunogenicity, and repeated injection can lead to IgE independent acute systemic allergic reactions in mice. Intravenous injection of c (RGDyK) liposomes can induce the production of specific IgG antibodies in mice. Reinjection can lead to the formation and deposition of immune complexes, trigger complement activation, and cause the release of allergic toxins and pro-inflammatory cytokines, leading to severe immune responses. On this basis, a method for determining the immune safety of targeted drug delivery systems using plasma IgG antibody titer detection was developed, and the immunogenicity of multiple targeted functional materials was screened using this method, resulting in several non immunogenic targeted peptide functional materials. At the same time, this method was used to evaluate the immunogenicity of different nano drug delivery systems, and it was found that the optimization design of targeted drug delivery systems can reduce the immune risk caused by the use of immunogenic targeted functional materials (Figure 10).
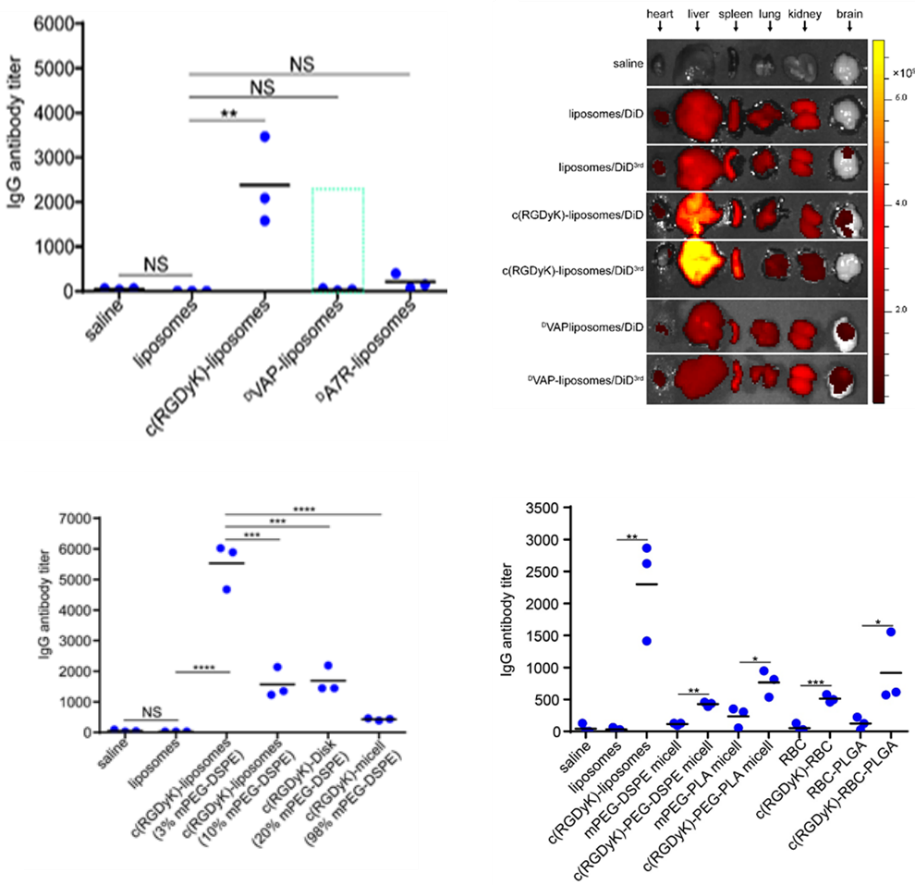
Figure 10. IgG antibody titer evaluation of immunogenicity of targeted drug delivery systems
Related patents:
Lu Weiyue, Wang Xiaoyi, Li Jinyang, Xie Cao; A molecular targeted drug liposome and its application in anti-tumor therapy (2019103925825, application date May 13, 2019)
 Home
>
Business
>
Tayzen
>
In vivo process evaluation techniques for targeted drug delivery systems
Home
>
Business
>
Tayzen
>
In vivo process evaluation techniques for targeted drug delivery systems










