Targeted PDC Drug Design and Evaluation Techniques
Peptide drug conjugates (PDCs), also known as peptide conjugated drugs, are composed of linkers, homing peptides, and cytotoxic payloads. homing peptides can specifically target protein receptors overexpressed on the surface of tumor cells, thereby transmitting cytotoxicity and inducing tumor cell apoptosis.
1.1 Targeted PDC drug design technology
Equipped with an advanced peptide screening technology platform, it can meet the needs of PDC for target peptides. At the same time, using targeted peptide molecule stabilization technology can further optimize target peptides; A technical platform has been established to facilitate the rapid optimization of linkers and the rational design of drug peptide molecule binding. Currently, we are developing PDC drugs for VAP peptides and doxorubicin, as well as PDC drugs for pVAP peptides and doxorubicin.
1.2 Targeted PDC drug evaluation technology
Evaluation techniques include characterization of physicochemical properties, amino acid sequence composition, drug dissociation release, molecular interactions, in vitro and in vivo targeting, in vivo pharmacokinetics and tissue distribution, and in vitro and in vivo pharmacodynamic evaluation.
Example: PDC drug VAP-DOX
By utilizing the affinity of VAP to the highly expressed GRP78 protein on the surface of tumor cells, the anti-tumor drug doxorubicin was targeted and delivered to tumor tissue, and the doxorubicin drug prototype was released through acid sensitive binding bonds in the slightly acidic environment of the tumor and acidic conditions of the tumor cell internalization process, significantly increasing the tolerance dose of doxorubicin and enhancing its anti-tumor effect (Figure 14).
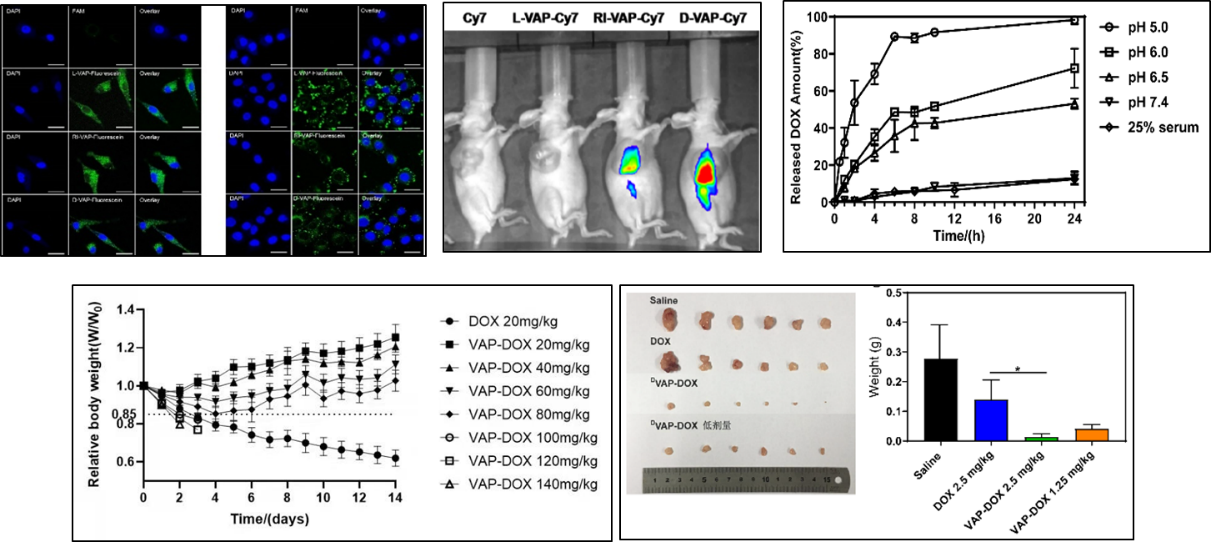
Figure 14. Characterization, safety, and efficacy of VAP-DOX
Related patents:
Lu Weiyue, Guo Haiyan, Xie Cao; A targeted functional molecule modified antibody complex, composition, and its use (PCT/CN2020/119919, application date October 9, 2020; 2019109601853, application date October 10, 2019)
 Home
>
Business
>
Tayzen
>
Targeted PDC Drug Design and Evaluation Techniques
Home
>
Business
>
Tayzen
>
Targeted PDC Drug Design and Evaluation Techniques








