Design and Evaluation Techniques for Bioadhesive Formulations
1.1 Design Technology of Bioadhesive Formulations
We have a technical platform based on the entanglement between medicinal excipient molecules and mucosal protein molecules, as well as the design and preparation of biological adhesive formulations based on molecular imprinting principles, as well as the promotion of mucus/mucosal penetration. Using this technology platform, acyclovir bioadhesive microspheres and amoxicillin bioadhesive microspheres were developed.
1.2. Evaluation Techniques for Bioadhesive Formulations
Evaluation techniques include: physicochemical properties, mucin interactions, cellular level pharmacodynamic evaluation, qualitative and quantitative analysis of in vitro and in vivo mucosal retention, qualitative and quantitative analysis of mucosal permeability, and pharmacodynamic evaluation of model animals.
Example: PEG modified docetaxel nanocrystals modified with TAT for vaginal mucosal delivery
Preparation of chemotherapy drug docetaxel into nanocrystals, PEG modification of nanocrystals based on dopamine polymerization coating technology to improve the hydrophilicity of nanoparticles, reduce the interaction between nanoparticles and mucin, and improve the ability of nanocrystals to penetrate the mucus barrier; And by modifying PEG modified docetaxel nanocrystals with cell penetrating peptide TAT, the performance of the nanocrystals in penetrating the mucosal barrier and tumor tissue was improved, achieving better anti-tumor effects (Figure 16).
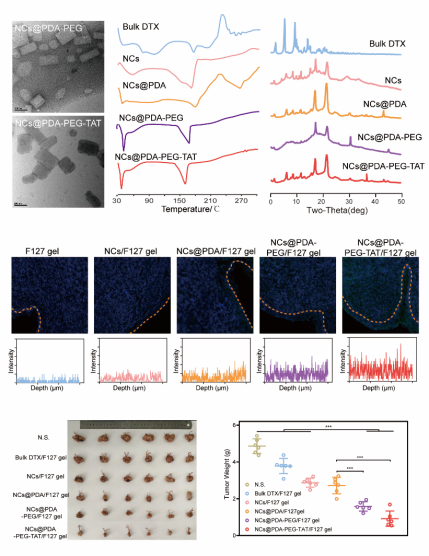
Figure 16. Characterization and efficacy of PEG modified docetaxel nanocrystals modified with TAT
Related patents:
1. Lu Weiyue, Liu Zhepeng, Pan Jun, Chen Lan, Zhang Yan, Li Baoguo; A preparation method for bioadhesive microparticle drug formulations (2004100180049, application date April 28, 2004)
2. Pan Jun, Liu Zhepeng, Lu Weiyue; A method for preparing gastrointestinal bioadhesive microsphere drug formulations (031512046, application date September 25, 2003)
 Home
>
Business
>
Tayzen
>
Design and Evaluation Techniques for Bioadhesive Formulations
Home
>
Business
>
Tayzen
>
Design and Evaluation Techniques for Bioadhesive Formulations








