In situ gel preparation design and evaluation technology
1.1 In situ gel preparation design technology
In situ gel preparation refers to a drug delivery system in which polymer materials are prepared and administered in solution state, and changes in biological phase or conformation occur at the application site due to changes in physiological environment, forming a viscous semi-solid. We have many types of ready to use gel technology platforms, such as ion sensitive, temperature sensitive and pH sensitive, which can be used to increase the local drug concentration, improve the drug efficacy, or improve the systemic bioavailability of drugs. Acyclovir ready to use gel for eyes was developed using this technology platform and applied for clinical research (clinical trial application No.: Shanghai 201001316).
1.2 In situ gel preparation evaluation technology
Evaluation techniques include: rheology, in vitro release, interaction with mucin, retention of lumen mucosa in vitro, quantitative evaluation of in vivo retention of living animals, pharmacodynamic evaluation of model animals, etc.
Example: potassium ion temperature sensitive instant gel
Using the potassium ion sensitivity and temperature sensitivity of natural polysaccharide carrageenan against HPV infection, the pharmaceutical excipient poloxamer and bioadhesive pharmaceutical excipient kapop, a ready to use gel that can flow freely at room temperature, inject into the vagina quickly semi-solid, effectively stay in the vagina and continuously release drugs, can increase the local bioavailability of drugs in the vagina by 50%, without significant irritation of vaginal mucosa; The instant gel can also improve the absolute bioavailability of the painkiller ketorolac tromethamine after nasal administration by nearly twice (65%, gel agent vs. 25%, solution agent) (Figure 17).
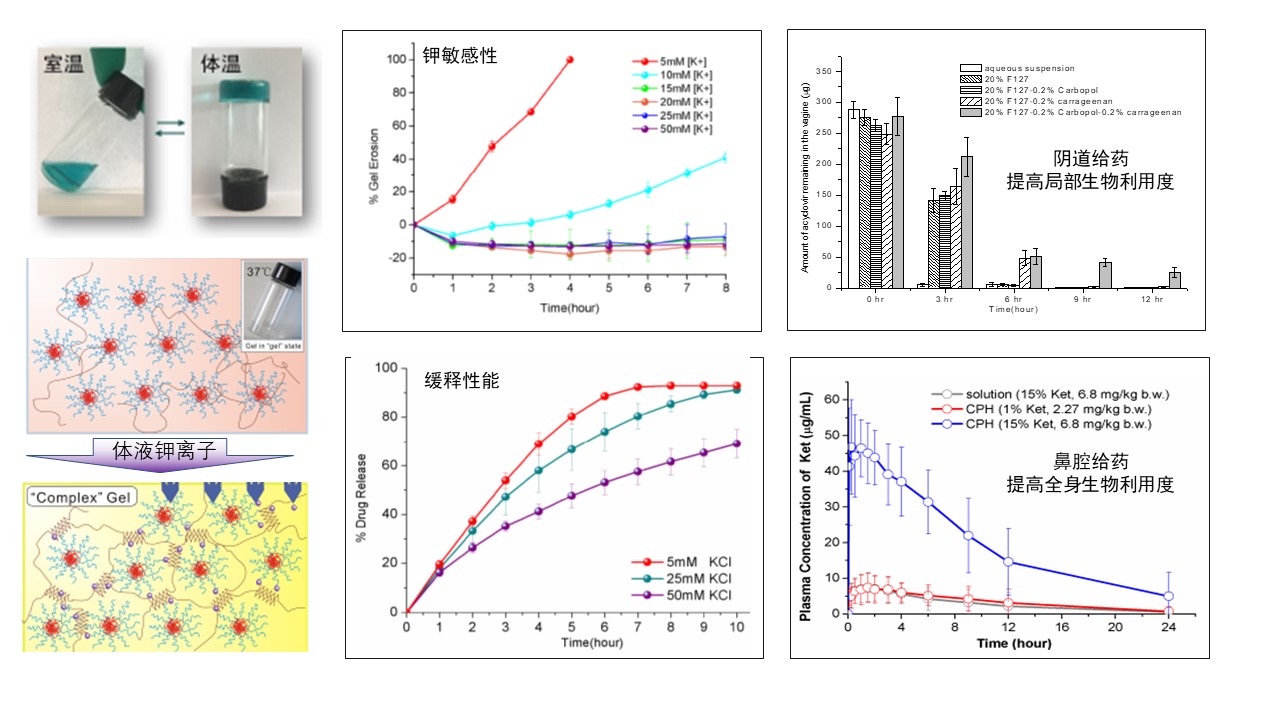
Figure 17. Characteristics and application of potassium ion temperature sensitive instant gel
Related patents:
Liu Yu, Wei Gang, and Lu Weiyue; A bioadhesive thermosensitive in situ gel sustained-release preparation for vaginal administration (2008102007160, authorized on August 6, 2012)
 Home
>
Business
>
Tayzen
>
In situ gel preparation design and evaluation technology
Home
>
Business
>
Tayzen
>
In situ gel preparation design and evaluation technology








