The upstream process development team has experience in developing different types of biological macromolecules, and can undertake more than 5 process development (including post process optimization) and 2 or more process characterization projects annually. The team members have a master's degree or above, and have an average of more than 5 years of experience in upstream related work. They have accumulated process development and characterization experience in over 20 projects.
The upstream process development laboratory is equipped with 20 Sartoris 2L&10L bioreactors to support the process development of multiple cultivation modes such as feed batching and N-1 enhanced process. It has various advanced process analysis equipment such as Vicell XR, Siemens blood gas analysis, high-throughput osmotic pressure analyzer and Cedex Bio biochemical analyzer, which can provide customers with services such as sample preparation and process development at different stages.

Service scope
 1. Service Case 1
1. Service Case 1
1.1 Background
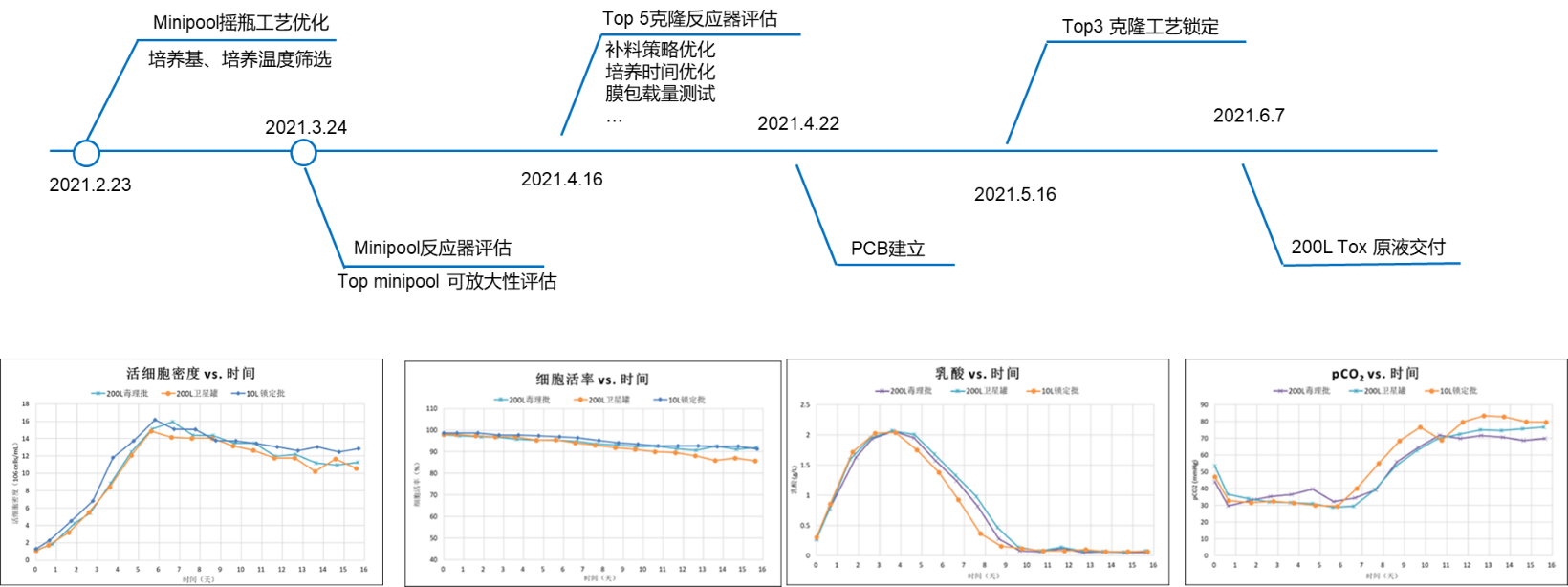
Timeline Urgent Development of Dual Antibody Molecular Processes
1.2 Challenges
The CLD team initiated a clone stability assessment and only three months remain until the delivery of the 200L toxicological batch stock solution requested by the customer
1.3 Solution/Results
The CCPD team conducted two rounds of process development using minitool in the early stage
Select the top 5 clone for process optimization to reduce the risk of project delay due to the uncertainty of final cloning due to the lack of cell line stability results
①Based on PDL30 pre stability evaluation data and historical project experience, the optimal clone (stability, yield, quality, and scalability) was successfully selected for process locking
②Staged technology transfer, seamless connection between 10L process locking and 200L production seed recovery
③The delivery of toxicological batch production was completed as scheduled, and the results of the 200L scaled up production were highly consistent with the performance of the small-scale trial process. The yield was>4.5 g/L, and the purity was>95%
2. Service Case 2
2.1 Background
Process optimization in the later stage of clinical phase II project
2.2 Challenges
Maximize expression while ensuring consistent product quality/
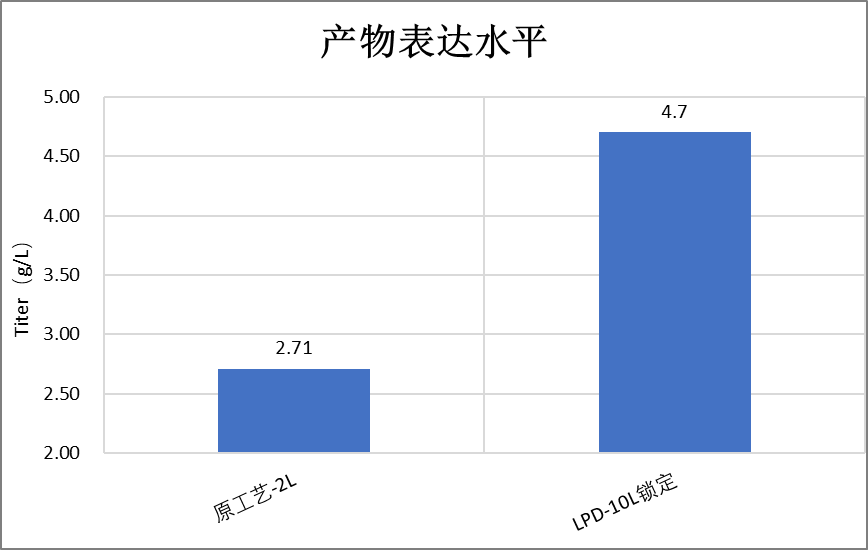
2.3 Solutions/Results
Sort out historical process development reports and determine the main direction for later process optimization
After optimization, the expression level reached 4.7g/L, compared to the original process of 2.7g/L, an increase of about 80%, and the product quality remained consistent with the original process
 Home
>
Business
>
Tarlead
>
Development of cell culture process
Home
>
Business
>
Tarlead
>
Development of cell culture process





 1. Service Case 1
1. Service Case 1





