The formulation process development team has experience in developing different types of biological macromolecules. The team members are diverse, with an average of more than 5 years of experience in formulation related work, and a total of over 10 project process development experience. Since 2021, multiple dual antibody formulations (including high concentration liquid formulations) have been developed, and three projects have successfully passed IND applications in China, the United States, Australia, and other regions, with a success rate of 100%.
The formulation process development laboratory is equipped with first-class formulation process development equipment, including Uncle, MFI, viscometer, desktop filling machine, etc., capable of undertaking projects at different research and development stages (more than 5 process development projects and 2 process characterization projects per year).

Service scope
1. Service Cases
1.1 Background
Development of Preparation Process for Double Antibody Project
1.2 Challenges
The project timeline is tight, with only two rounds of screening time and low fault tolerance
1.3 Solution/Results
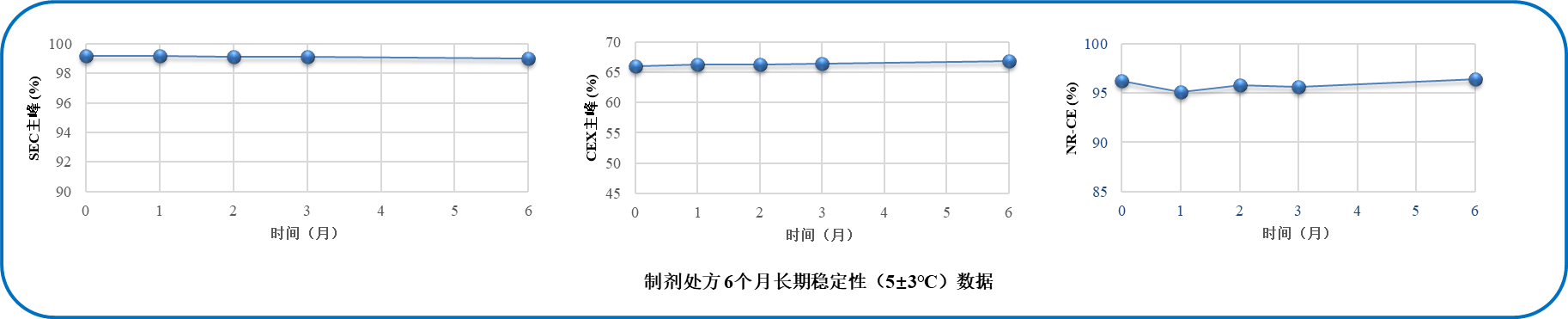
Based on the team's prescription development experience, optimize the screening process reasonably, and take about 11 weeks (industry development time is generally 3-5 months) to select suitable formulation prescriptions.
The prescription confirmation results indicate that the selected formulation has good stability and has successfully completed the IND application.
2. Service Case 2
2.1 Background
Change in concentration of Shuangkang injection preparation
2.2 Challenges
Increase the protein concentration of Shuangkang injection from 5 mg/mL to 150 mg/mL, and develop a high concentration subcutaneous injection formulation
2.3 Solutions/Results
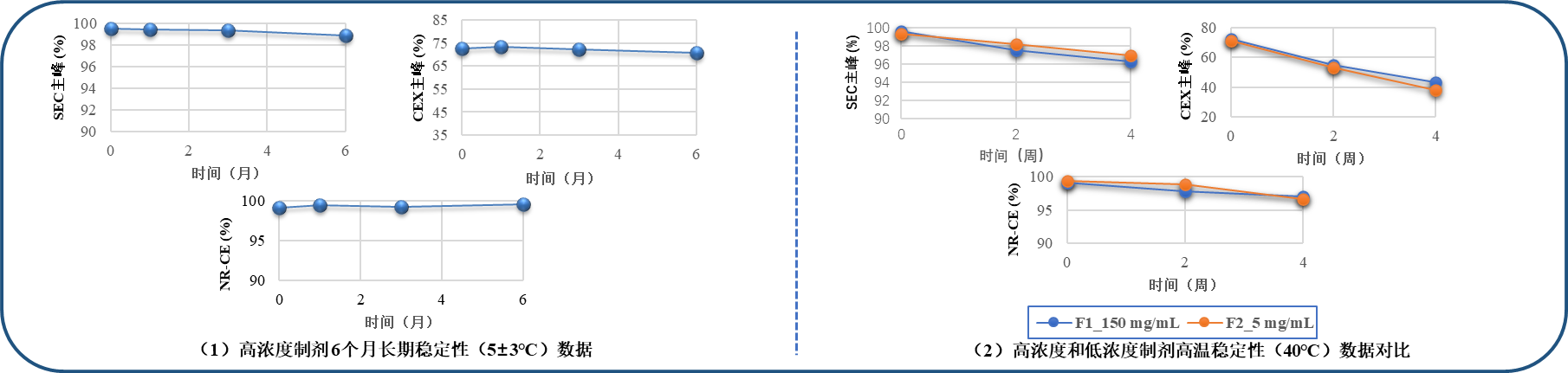
Sort out historical process development reports and determine appropriate prescription development strategies based on the team's experience in developing high concentration formulations.
The prescription confirmation results indicate that the high concentration formulation after the change has good stability; And under high temperature conditions, there is no significant difference in the degradation rate of various purity indicators compared to the original low concentration formulation.
 Home
>
Business
>
Tarlead
>
Preparation process development
Home
>
Business
>
Tarlead
>
Preparation process development












